Acetals and Ketals – Functional Group Spotlight
Acetals and Ketals have a special place in my OChem-loving heart! Not least because I spent most of my PhD trying to make spiroacetal structures that were part of a natural product called spongistatin. In fact, I like them so much that my last major research project before leaving academia was about trying to synthesize spiroacetals via a new synthetic route. More about that another day! Today, we have another Functional Group Spotlight by MedChemProf, after the popularity of his Amide Functional Group Spotlight. Read on and be sure to have a play with the cool interactive molecules scattered throughout the post! – Mark
Acetal, hemiacetal, ketal or hemikal – they’re more than just aldehyde and ketone siblings
In general, I have the impression that the Acetal and Ketal functional groups are often presented to students as only special cases of their carbonyl containing siblings – the Aldehydes and Ketones. Most likely this is simply because, pedagogically, it makes sense to first describe the extensive chemistry of the Aldehydes and Ketones prior to deriving what ends up seeming like secondary functional groups. As an unfortunate consequence of this however, I find that many students who have taken Organic Chemistry and then transition into fields of study such as Pharmacy, Medicine (or even some of the Biological sciences), fail to recognize the groups and their importance to either Drug Metabolism or the structure and reactivity of Carbohydrates (Sugars). With these thoughts in mind, let’s take another look at Acetals and Ketals and then work our way to highlighting their importance in a greater biological context.
| FUNCTIONAL GROUP | 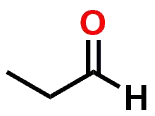 Aldehyde | 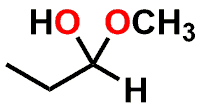 Hemiacetal | 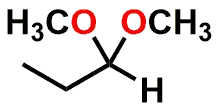 Acetal |
| Oxidation State of Carbon in Functional Group | +1 | +1 | +1 |
| Hybridization State of Carbon in Functional Group | sp2 | sp3 | sp3 |
| FUNCTIONAL GROUP | 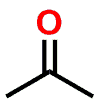 Ketone | 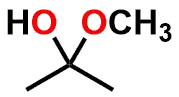 Hemiketal | 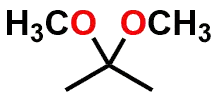 Ketal |
| Oxidation State of Carbon in Functional Group | +2 | +2 | +2 |
| Hybridization State of Carbon in Functional Group | sp2 | sp3 | sp3 |
Figure 1: Acetal & Ketal examples shown alongside their Aldehyde & Ketone precursors. Also illustrated are the half-way (hemi-) points between the functional groups which are known as either a Hemiacetal or Hemiketal. Notice that there is no change in Oxidation State within each series.
If you examine Figure 1, you’ll note that the Aldehyde and Ketone molecules are identical to the Acetal and Ketal counterparts when considering: a) the carbon backbone of the molecules and; b) the oxidation state of the carbon that is part of the aforementioned functional groups. The most significant differences between the groups is that the carbon is sp2 hybridized and trigonal planar in shape for the Aldehyde and Ketone (1 Carbon-Oxygen double bond), but sp3 hybridized and tetrahedral in shape for the functional groups that contain a central Carbon with two Carbon-Oxygen single bonds.
While the difference in hybridization may at first seem to be a considerable, it should be kept in mind that the two groups are actually related by an equilibrium. It is this equilibrium that governs much of the structure and reactivity of carbohydrates and also the metabolic decomposition of drug molecules following their oxidation by Cytochrome P450 enzymes. Before we look at some specific examples, lets review the general mechanism of the interconversion of an aldehyde to an acetal (or ketone to ketal).
The terms ‘acetal’ and ‘ketal’ have had varied use over the years. Back in the day, acetals were diethers of geminal diols (ie. 1,1-diols), with a formula R2C(OR’)2 ( R’ ≠ H ) where at least one R = H, and were therefore derived from aldehydes. Whereas, when neither R = H, they were called ketals and were obviously derived from ketones. Then, IUPAC decided that all compounds with structure R2C(OR’)2 ( R’ ≠ H ) were to be called acetals, regardless of whether they were derived from aldehydes or ketones. Chaos ensued! OK, just kidding… but it was confusing, with most Professors continuing to teach the old system of acetals and ketals. More recently, IUPAC have compromised and now all compounds with formula R2C(OR’)2 ( R’ ≠ H ) are acetals, but the subclass of these that are derived from ketones may again be called ketals, having been reinstated as a subclass of acetals! (Source: IUPAC Gold Book)
Mechanism of hemiacetal and acetal formation
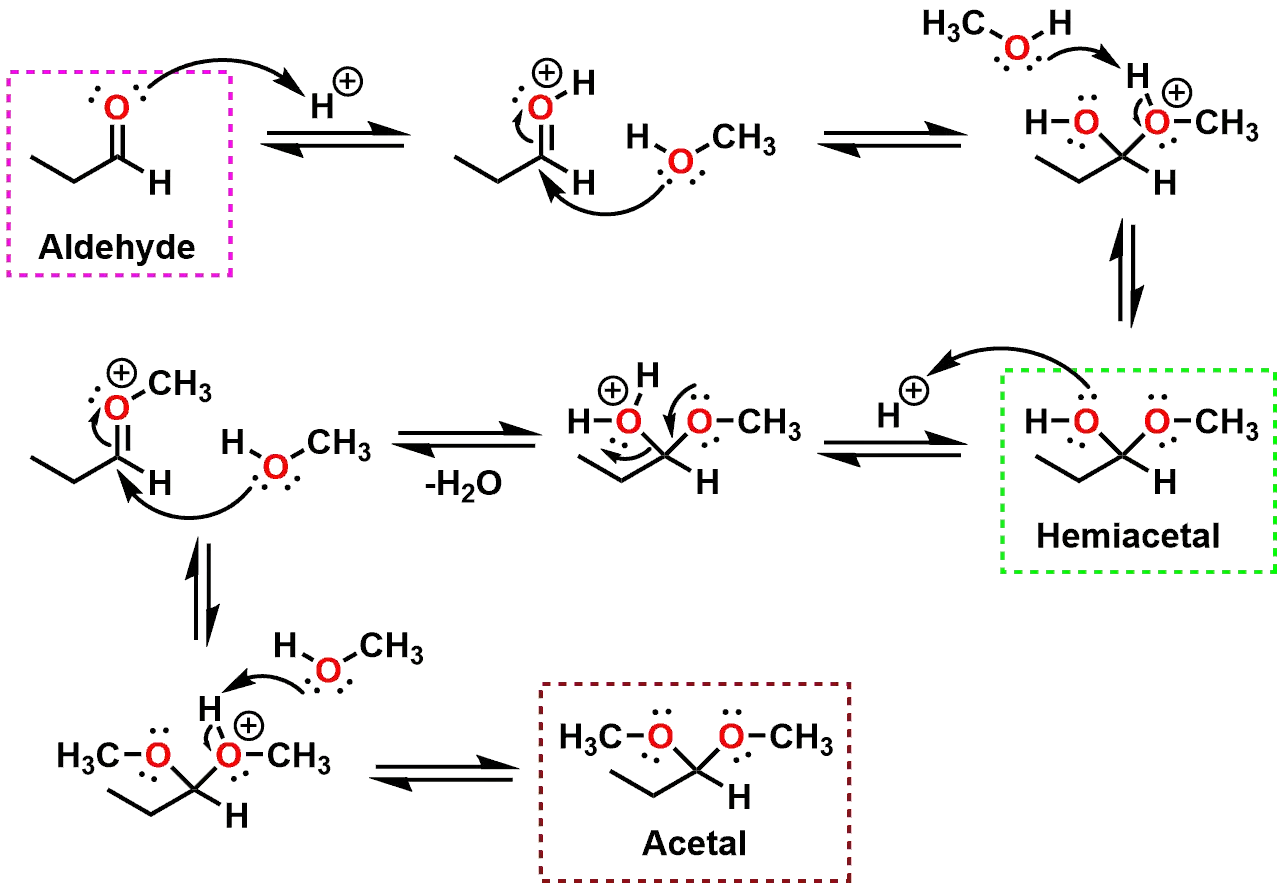
The above mechanism (Figure 2), showing the equilibrium between an Aldehyde and an Acetal, illustrates all of the steps necessary for the interconversion to take place. The same general mechanism can be found in any sophomore Organic Chemistry textbook. A better understanding of the mechanism may be possible if, instead of focusing on the arrow-pushing, attention is directed towards the mechanistic steps in the context of the surrounding conditions where the reaction is taking place.
An undergraduate or graduate student in Chemistry may indeed carry out the above reaction in a round bottom flask using Methanol as the solvent in order to convert an aldehyde to an acetal as part of a Protecting Group strategy. Acetals are less susceptible to nucleophilic attack as compared to the corresponding Aldehyde and can therefore be carried along in a protected state during a multi-step synthesis. Eventually, the aldehyde could be unmasked at a later stage by taking advantage of the equilibrium and hydrolyzing the acetal back to an aldehyde. In the context of a biological system, however, the presence of excess methanol would not make sense since any biological system is mostly comprised of water. Keeping this in mind, the hydroxyl group role in the mechanism would instead be played by alcohol groups from a sugar, a hydroxyl from a side-chain of an amino acid, or from an oxygen from a molecule of water itself.
Sweet, sweet chemistry – Sucrose has both acetal and ketal functional groups
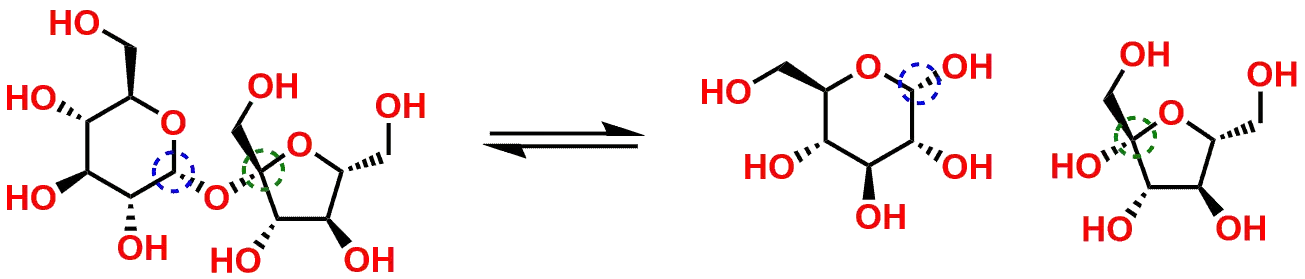
Let’s look at the sugar Sucrose (Figure 3 and Figure 4) as an example to illustrate some of these mechanistic principles. Sucrose is a disaccharide comprised of the combination of the monosaccharides Glucose and Fructose. If you examine the structure in Figure 3, you will see the Glucose on the left side in its pyranose (six-membered ring) form, connected via an Oxygen bridge to the Fructose on the right in its furanose (5-membered ring) form. There are two Carbons circled in the Figure 3 that highlight the centers as an Acetal (circled in Blue) and a Ketal (circled in Green). Please note, while these two centers are Acetal and Ketal functional groups, they are also referred to as Anomeric Centers due to the fact that that are part of a carbohydrate molecule. For our purposes however, we will refer to the two Carbons using the Acetal and Ketal notations.
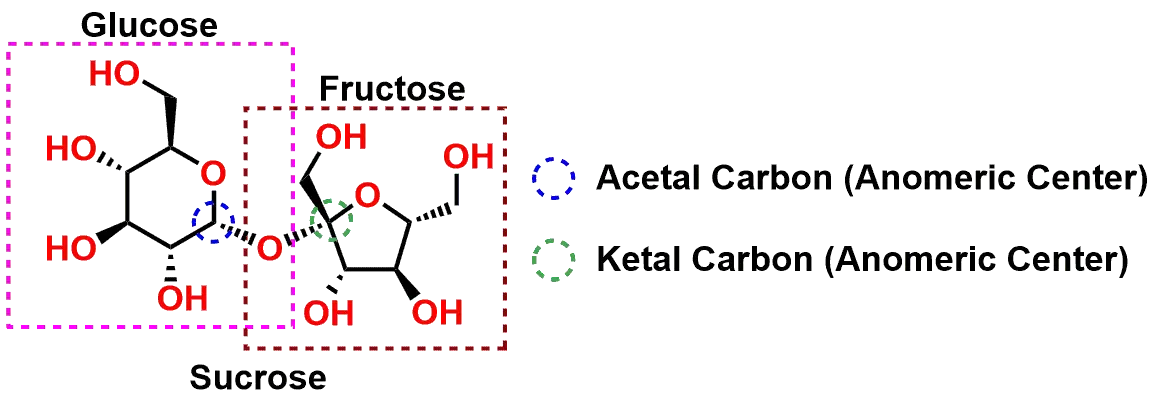
Hydrolysis of sucrose yields glucose and fructose
The highlighted centers within Sucrose can be enzymatically hydrolyzed (through a mechanism analogous to the reverse of the one shown in Figure 2) to yield the product sugars in their cyclic pyranose and furanose forms which should be familiar to students (Figure 5). You will also note that the result from the hydrolysis of Sucrose yields the sugar products in their Hemiacetal and Hemiketal forms.
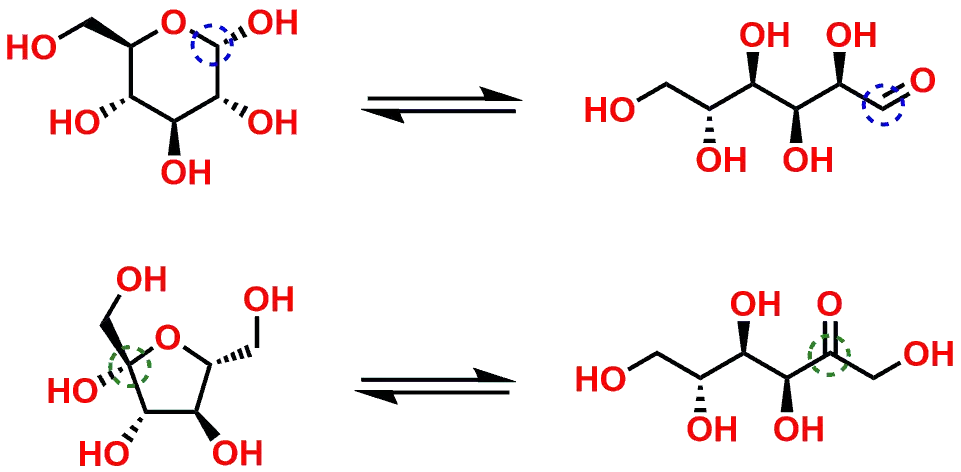
Once the sugars are liberated from their Acetal / Ketal forms to their Hemiacetal and Hemiketal state, they are then capable of further equilibration to ultimately adopt their corresponding Aldehyde and Ketone personas. Figure 6 illustrates the equilibria between the sp3 hybridized Hemiacetal/Hemiketal forms to the sp2 hybridized Aldeyhde/Ketone forms.
Invert Sugar
Enzymes aren’t the only catalysts that facilitate hydrolysis of sucrose! Have you ever heard of ‘invert sugar’? Some recipes call for heating sucrose in water with a little lemon juice (acid catalyst!). This hydrolyzes the sucrose to give equal parts glucose and fructose. The term ‘invert’ comes from the fact that the sign of the optical rotation ‘inverts’ from positive to negative, since [α]D20 for each sugar is as follows:
D-Sucrose = +66
D-Glucose = +52
D-Fructose = −92
Source: Wikipedia
Acetals, hemiacetals, ketals and hemiketals in drug metabolism
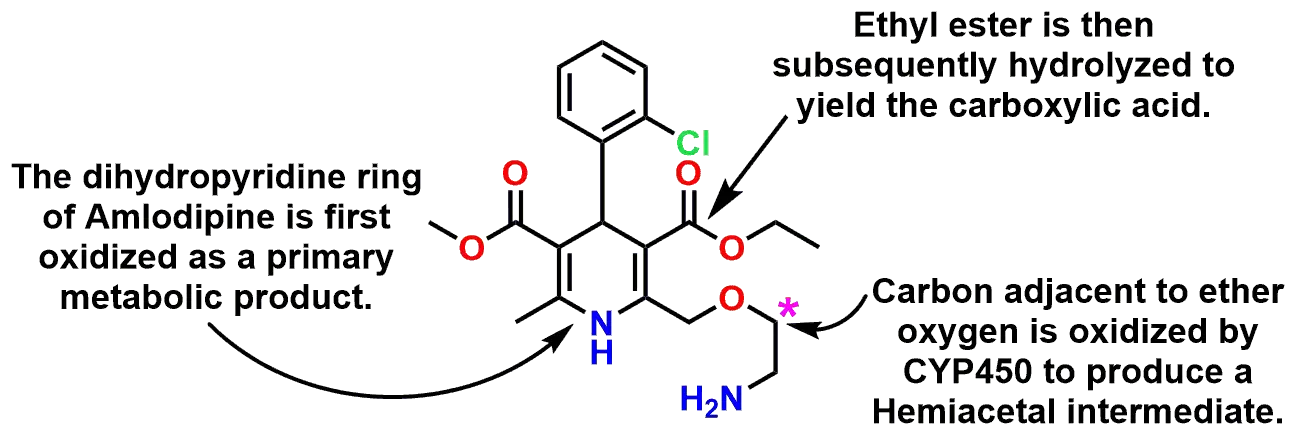
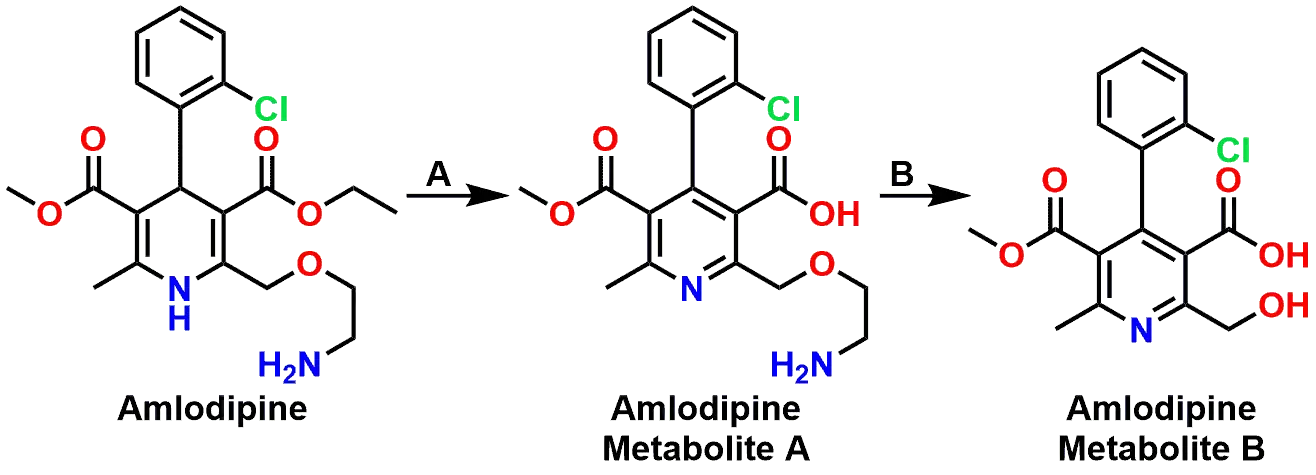
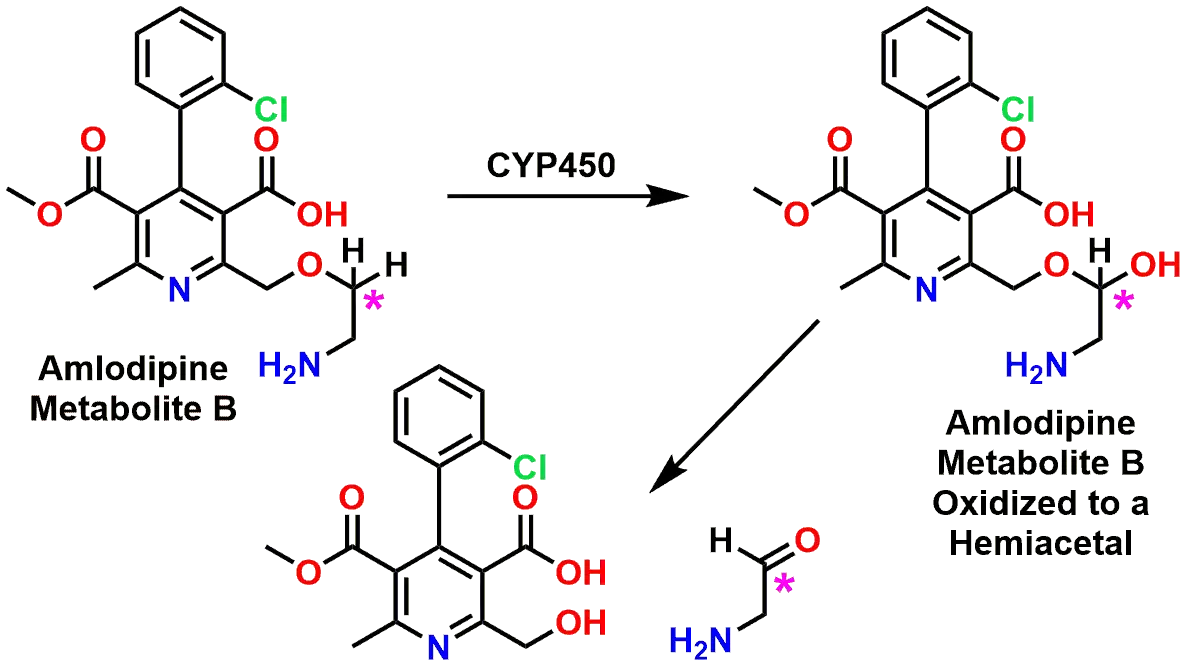
The equilibrium between the carbonyl forms of aldehydes or ketones and their associated acetal/hemiacetal (or ketal/hemiketal) forms also plays a critical role during the body’s metabolism of xenobiotics (drugs). As an example, the drug Amlodipine (marketed under the name Norvasc®) is a calcium channel blocker and is used to treat high blood pressure. Some of the principal metabolic reactions that Amlodipine undergoes is oxidation of the dihydropyridine ring, hydrolysis of the ethyl ester, and oxidative dealkylation of the ethoxyamine side chain (Figures 7 and 8). It is the latter of these reactions that will be highlighted due to the presence of a Hemiacetal as an intermediate in the oxidative dealkylation. These three reactions can be seen stepwise in Figure 8 and how each of the three distinct processes results in degradation of the drug molecule.
P450 oxidation to a hemiacetal, then hydrolysis
If we focus on Step B in Figure 8, we can explore how the oxidative dealkylation works and how a Hemiacetal is involved. If you examine Figure 9 starting from the Metabolite B, you can see that following the oxidation of the Carbon (marked with asterisk) by a Cytochrome P450 enzyme, the resulting product is a Hemiacetal. Many students mistakenly believe that the Cytochrome P450 is responsible for the second step where the Hemiacetal then equilibrates to the Aldehyde and Alcohol final products. In reality, the Cytochrome P450 is only responsible for the oxidation step and then the regular equilibrium processes of a Hemiacetal-Aldehyde takes over in the aqueous environment. The side chain falls off as the Aldehyde by-product, leaving behind the metabolized drug.
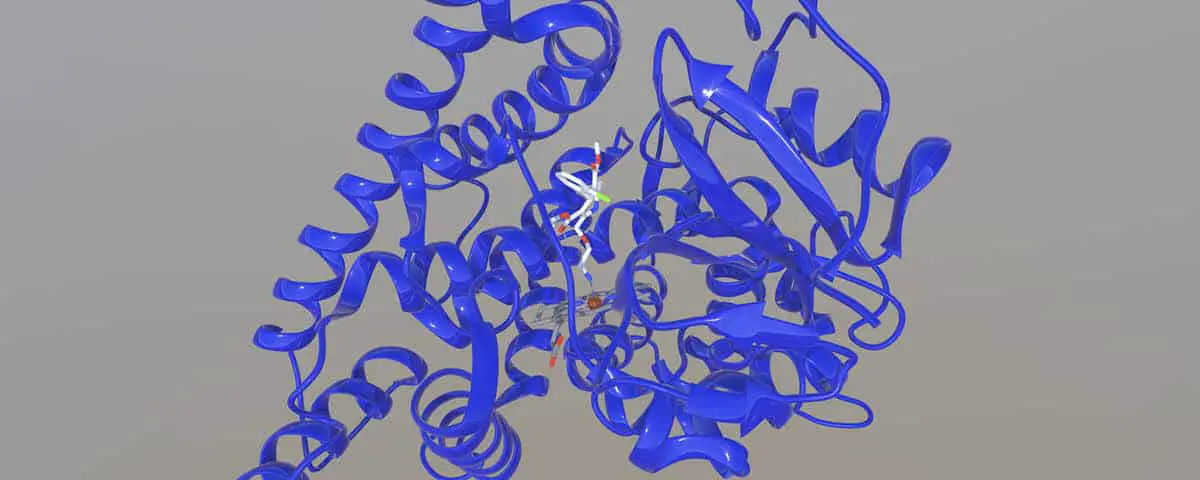
The mechanism of the fragmentation is simply the reverse of the hemiacetal formation in Figure 2. As a point of interest, please also examine the 3D model of Amlodipine bound to the heme of a cytochrome P450. The model itself does not depict the active oxidative state of the heme, but it does illustrate the general proximity necessary for a drug to be in regards to the heme portion of the enzyme, for oxidation to take place.
Now, what about nitrogen…
While it is a little too much to cover in this post, as a though experiment, think about all that we just discussed about Acetals and Ketals and then substitute a Nitrogen atom for one of the Oxygen atoms. If we did that, we would be venturing into the field of Hemiaminals (acetals that contain an amine) and very similar chemistry would result. Maybe for another blog post…
Mark here again – I hope you found that a good read. I’ve gotta say, I love the 3D models and I can never have enough acetals and ketals!
If you liked this Spotlight on Acetals and Ketals by MedChemProf, then be sure to also check out his Spotlight on the Amide Functional Group!
Leave a comment below if you have any questions or to suggest the next topic for a Spotlight article.




You have explained many tough organic concepts in easy way. It would be great if we could get the references too for citing these discussion.